Blog about new medical equipment
Blog about new medical equipment
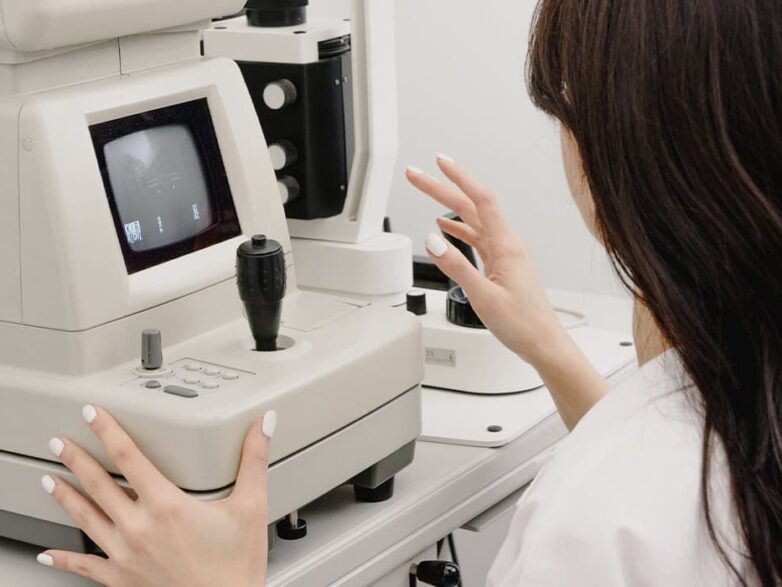
LASERS FOR EXCIMER LASER VISION CORRECTION
New Medical Equipment

Today, laboratory equipment for medicine enjoys increased attention and considerable demand, and this is not surprising at all.

Vacuum equipment is often used in laboratories, especially those performing electrophysical research.

Vacuum systems (tubes) are used for blood sampling for analysis. Modern production technology guarantees high quality of the finished product.
Today, ultrasound scans are one of the most popular medical diagnostic methods. Ultrasound scanners are firmly established in every modern clinic

One of the current trends in the development of diagnostic equipment is the development of devices and instruments that can be used by patients who have no medical training.
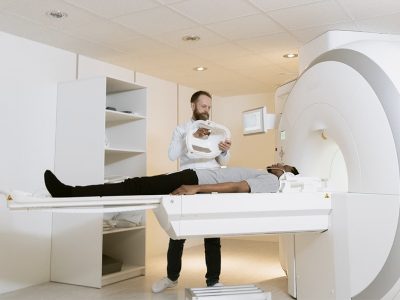
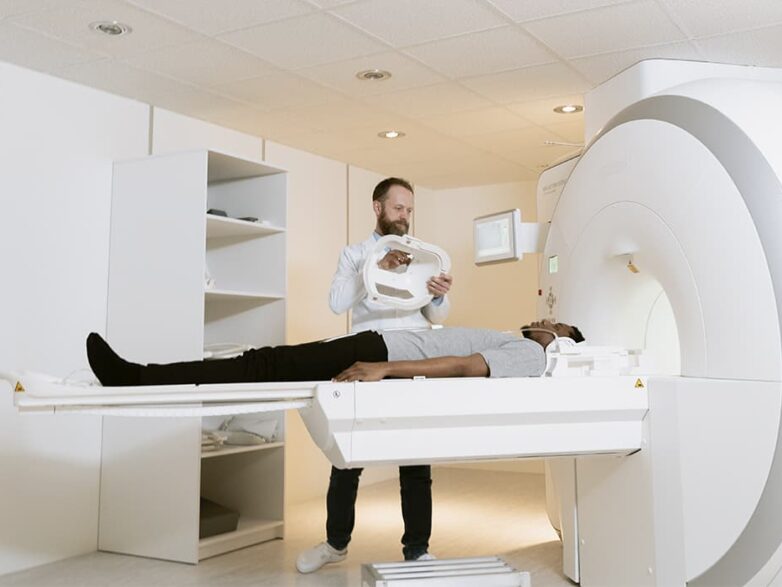
Thanks to the modern CT scanner, medical professionals can now examine every part of the human body.
Hi everyone, my name is Dorothy Davis. I am a medical device salesman with a medical background and love to explore new things. In my blog you will learn what new medical equipment is on the market right now, what the equipment is for, and much more.
About blog
What is medical equipment

MEDICAL EQUIPMENT: VIEWS AND FEATURES
A doctor’s main tool is his or her sharp mind. But, no matter how sharp your mind is, you will perform surgeries with a scalpel or a radio wave knife.
What is medical equipment
PRINCIPLE OF OPERATION OF ELECTROSURGICAL UNITS
Nowadays, surgery using high-frequency electrosurgical devices is one of the modern technologies
What is medical equipment
TYPES OF MODERN SURGICAL EQUIPMENT
Equipment for operating rooms and intensive care units can be classified according to their purpose: general surgical equipment – these are surgical instruments
Our Suppliers
 Best Online Casino GGBet: Official Club Evaluation Criteria
Best Online Casino GGBet: Official Club Evaluation Criteria
 Step into the vibrant universe of online casinos at NettCasino.com; your gateway to unparalleled gaming and exhilarating moments!
Step into the vibrant universe of online casinos at NettCasino.com; your gateway to unparalleled gaming and exhilarating moments!
 Experienced, caring and professional dentist in Surrey, BC. We serve adults and children, installing crowns and veneers.
Experienced, caring and professional dentist in Surrey, BC. We serve adults and children, installing crowns and veneers.
 Looking for non-stop action and endless thrills? Dive into the heart of the action at casino without Swedish license, and get ready to ride the waves of fortune at utansvensklicens.casino
Looking for non-stop action and endless thrills? Dive into the heart of the action at casino without Swedish license, and get ready to ride the waves of fortune at utansvensklicens.casino